Protein has a love hate relationship with brewers. We love the work they do for us, but getting them to leave can be a nuisance! Protein is the generic word for a string of amino acids (amino acids are the physical building blocks for all living things) that fold to create functional blobs of amino acids in the simplest sense. An exclusive class of proteins, enzymes, performs or catalyzes all kinds of chemical reactions from the breakdown of alcohol in your liver to the conversion of starch to sugar in malt! Proteins also create body and mouthfeel in a beer leaving the weak and thin beers in the dust. Graduating from the milk mustache of grade school, proteins help form the foam of your uniquely adult beer mustache!
In the lab, there are several methods to test for proteins. We have a quick and easy enzyme test that can be performed in 15 minutes that can confirm if enzymes are present. We can titrate for alpha amylase enzymes. We can titrate for diastatic power which will measure both beta amylase and alpha amylase. We can optically measure the amount of soluble protein after a mash. We can also measure the total protein by combustion.
Each of these methods tell you something different about the protein in your malt or measures a specific type of protein in your malt. Looking at the total protein, typical malts are less than 14% protein. Total protein measurements have evolved through the years. Taking a trip down the Briess memory lane, we visit the Kjeldahl method using what looks like a mad scientist’s laboratory!

Before the fine art of automated laboratory equipment, the finely tuned fingers of a white coated scientist fiddled with glassware, acids, bases and knobs. Danish chemist Johan Kjeldahl developed an early method of what is now known as the Kjeldahl method and the standard to measure new protein analysis methods by. His method measures total nitrogen in a sample, which may result in a slightly inflated protein result, if appropriate standards for each type of sample tested are not applied.

The sample to be tested, in our case malt, is digested or broken down in a specific flask with concentrated acid in the presence of a catalyst like copper and potassium sulfate. The copper will encourage the reaction to take place and speed up the reaction; the potassium sulfate will not participate in the reaction but increase the boiling point of the reaction or increase the temperature of the reaction before evaporation takes place (think how salt decreases the melting point of ice, ice normally melts at 32°F, with salt it can melt at lower temperatures or before the day gets warm enough to melt the sidewalk ice alone). The malt is broken down releasing carbon dioxide, water and ammonium in solution. The reaction pH is increased, allowing the ammonium in solution to become ammonia gas and separate from the reaction solution. The ammonia gas is captured and dissolved into a known amount of acid. Ammonia is a base and neutralizes acid. Or the pH becomes less acidic as a ratio of ammonia neutralizes a ratio of acid. The left-over acid can be titrated by a base. The amount of base needed to neutralize the remaining acid is the amount of acid that was not neutralized by the ammonia. This can be calculated and subtracted from the original amount of acid, the ammonia yield. The ammonia yield can then be calculated to the actual mass or grams of protein with a product or sample specific nitrogen to protein conversion factor. This can be compared to the original amount of sample to get a percentage of protein in the sample.
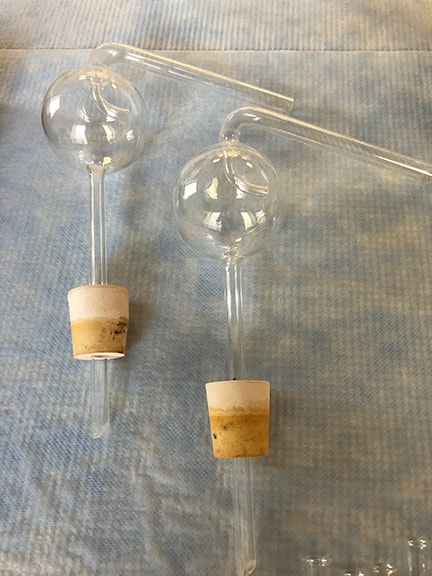
Our Kjeldahl set-up was probably purchased in the 1920s. Only one scientist remembers using this system in the Manitowoc lab, Randy Schmitz. His comments testify to the potentially hazardous conditions, he remembers knowing that the reactions were ready when the glassware would bounce! “…there’s not much movement in the liquid prior to the flasks “bumping” – which makes it even more difficult to determine when it would happen. We would usually set a timer for an approximate time at which they would be close to finished,” Randy comments about the Kjeldahl method.

This process is glassware intensive, labor intensive and not very friendly to the scientist performing the method with all the strong acids, bases and ammonia gas! As Randy knows, “you really need to be on your toes and have your wits about you when working with Kjeldahl, or things could turn out badly!” Though the original gold standard for protein analysis and very impressive to look at, our lab uses a new, rapid total protein analysis method.

Fast forward to the future and current state of Briess laboratories, combustion analysis based on the Dumas method developed by French chemist Jean-Baptiste Dumas measures total nitrogen in a matter of minutes. The combustion chamber heats the pre-weighed sample, ground malt, in the presence of copper oxide driving off carbon dioxide, water, nitrogen and nitrogen oxides. The oxides are reduced to gaseous nitrogen after running overheated copper. The carbon dioxide is absorbed by a potassium oxide solution. The remaining nitrogen gas is quantified and converted to a measurement of protein. After the initial combustion, the reaction takes place in a column packed with differing reagents. Packing this column can be time-consuming and some of the chemicals can be harsh. As with the Kjeldahl method, this method measures total nitrogen, product-specific conversion factors should be applied to account for non-protein based nitrogen.
This method is much more user friendly in the sense that we are not boiling acids or connecting tubing that may leak ammonia gas! Additionally, the Dumas method can be completed within minutes with little to no prep or clean-up whereas the Kjeldahl method takes substantial chemical prep time, one to two hours to perform, and substantial glassware clean-up time. Science has really come a long way!
We are looking to sell our Kjeldahl set-up or glassware to make room for more awesome science in our lab. If you or anyone you know is in the market for glassware with history or a fully functional Kjeldahl system, please contact Randy Schmitz via contact us to learn more!



